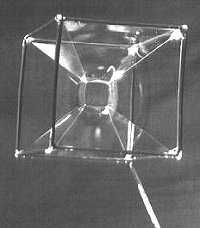
The soap bubbles represents a very interesting topic because they attract everyone's attention thanks to their charm as well as they are suitable to be the subject of many considerations in the different fields of physics, chemistry, optics and mechanics.
It is necessary to prefix a note about the general properties of liquids in order to understand a soap bubble's behavior. Every molecule of a liquid is subject to an attractive force from the surrounding molecules within a 100 nm of radius approximately. While the resultant of forces is null for the internal molecules (for symmetry reasons), concerning the superficial molecules the resultant is direct to the interior of the liquid.

|